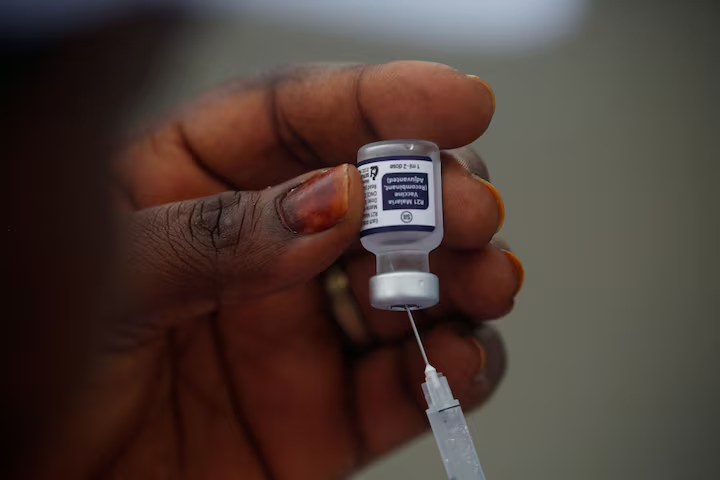
Nigeria has received its first batch of 846,000 doses of the R21 malaria vaccine from Gavi, the Vaccine Alliance.
The official launch took place on Thursday in Abuja, attended by officials from the Ministry of Health, the National Primary Health Care Development Agency (NPHCDA), and development partners.
Health Minister Ali Pate described the arrival of the vaccines as a significant milestone in the country’s efforts to eliminate malaria.
He said the vaccines are an opportunity for every child to live a life free of the disease.
Malaria is transmitted from human-to-human through the bites of female Anopheles mosquitoes. The infection cycle begins when elongated and highly mobile forms of the parasite, called “sporozoites”, are injected from the mosquito’s salivary glands into a person’s blood when the mosquito takes a meal.
The R21/Matrix-M malaria vaccine, developed by the University of Oxford and manufactured and scaled up by the Serum Institute of India (SII), is only the second vaccine the world has seen for a disease that has caused untold suffering for millennia.
The R21/Matrix-M vaccine targets the plasmodium ‘sporozoite’, which is the first form of the malaria parasite entering the human body.
The vaccine targets the first stage of the parasite’s life cycle
When the anopheles mosquito that carries the malaria parasite bites a person, it sends the parasite through the bloodstream, where it shapeshifts through stages of its life cycle. The complexity of the malaria parasite’s life cycle has meant vaccine development has been hampered for years. The R21/Matrix-M vaccine targets the plasmodium ‘sporozoite’, which is the first form of the malaria parasite entering the human body.
Only a few (10–100) sporozoites are injected by infected mosquitoes before the parasite multiplies, making them the ideal target for a vaccine. R21 is a subunit vaccine that delivers parts of a protein secreted by the sporozoite that are bundled up with a part of the hepatitis B virus that is known to trigger a strong immune response.
The vaccine also contains Novavax’s Matrix-M, an “adjuvant” which boosts the immune system response to make it more powerful and long-lasting. Vaccines work by putting the antigen, which is the piece of the virus or bacteria that our system recognises and responds to, in front of our immune cells. This technology – that was used in Novavax’s COVID-19 vaccine – induces the influx of antigen-presenting cells at the injection site and enhances antigen presentation in local lymph nodes, which means that the immune system is triggered as strongly as possible.
In regions where malaria transmission is highly seasonal, occurring primarily for 4 or 5 months each year, the R21 vaccine has displayed significant effectiveness. It was found to reduce symptomatic malaria cases by 75% in the year following a 3-dose series. Furthermore, the sustained efficacy of the vaccine was maintained with a fourth dose administered a year after the third. This high level of effectiveness is comparable to the efficacy demonstrated when using the RTS,S vaccine seasonally.
Additionally, when the R21 vaccine is administered based on age, it still demonstrates substantial efficacy, with a 66% reduction in malaria cases during the year following the first 3 doses. Again, a fourth dose given a year after the third dose maintains this efficacy.
The R21 malaria vaccine is the need of the hour
According to the latest World Malaria report, there were 247 million cases of malaria in 2021 with an estimated 619,000 malaria deaths globally, with the majority occurring in Sub-Saharan African countries. Children under the age of five accounted for about 80% of all malaria deaths in that region. Meanwhile, India contributes 1.7% of malaria cases and 1.2% of deaths globally. Also, over the last two peak years of the pandemic (2020–2021), COVID-related disruptions spiked 13 million more malaria cases. The R21 malaria vaccine will be hailed as a rare ray of light in a fight over many years.